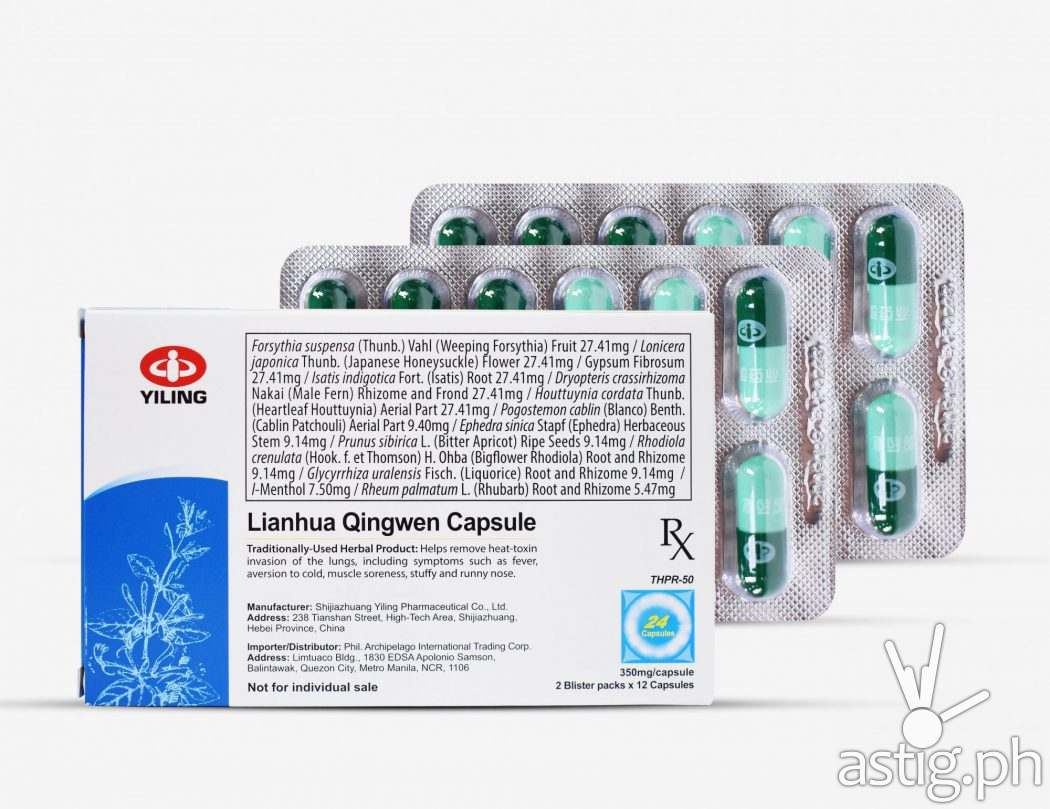
The popular traditional Chinese medicine (TCM) Lianhua Qingwen capsule is officially launched in the Philippine market through the efforts of Yiling Pharmaceutical and its exclusive Philippine distributor, Philippine Archipelago International Trading Corporation. Making it the first TCM to be registered as a traditionally used herbal product in the Philippines.
The Food and Drug Administration (FDA) approved the landmark registration of Lianhua Qingwen capsule in early August. The drug is widely used in China as part of its standard coronavirus treatments.
During the product launch, Olivia Limpe-Aw, the President of Philippine Archipelago International Trading Corporation stated that “The COVID-19 Pandemic has brought to the fore the importance and significance of TCM and the significant role of Lianhua Qingwen in particular in the prevention, treatment and control of this global epidemic.”
Lianhua Qingwen Capsule has been part of China’s standard therapy for mild and moderate coronavirus patients. It’s listed as a recommended medicine in the Diagnosis and Treatment Scheme of Novel Coronavirus Pneumonia (Versions #4-#8), issued officially by Chinese authorities.


The results of a prospective, randomized, controlled, and multi-center clinical study on treating COVID-19 with Lianhua Qingwen Capsules, which Chinese Academicians Zhong Nanshan, Li Lanjuan, and Zhang Boli and other experts worked on together with over 20 hospitals, were published in Phytomedicine, the journal with a high impact factor in the field of international herbal medicine. It’s found in the study in terms of clinical use that Lianhua Qingwen proved to be both safe and effective in treating COVID-19 in conjunction with conventional therapy as it could significantly relieve clinical symptoms of COVID-19 such as fever, debilitation, and cough, greatly improve pulmonary lesions, shorten the duration of symptoms, and increase the clinical recovery rate.
“It is our sincere hope that Lianhua Qingwen’s entrance into the Philippine market will contribute to the fight against the spread of COVID-19 in this country,” Yunling Zhang, VP of the drug’s manufacturer, Yiling Pharmaceutical, said during the launch. “It helped millions of patients during the coronavirus outbreak in both China and other countries.”
As of now, Lianhua Qingwen Capsule has been approved in nearly 20 countries and regions including Canada, Brazil, Romania and Thailand. In Kuwait, the medicine has been for the first time granted permission in countries other than China to treat symptoms caused by mild and moderate cases of COVID-19.
According to local TCM expert, Dr. Philip Niño Tan-Gatue, COVID-19 is new but the principles for treating it are not. Chinese medicine focuses, not just on the causes of the disease, but also the clinical or presentation of a disease at a particular point in time. COVID-19 is looked at the same way as SARS, or any other rapidly transmissible infectious disease. So long as a particular clinical pattern is recognized, then a formula used thousands of years ago can be used for modern diseases, albeit with modifications.
In the Philippines, however, despite its safety, Lianhua Qingwen Capsule is a prescription Rx drug requiring a prescription from a doctor holding a PDEA S2 license. Once obtained, prescription-holding consumers may purchase leading drug stores at a suggested retail price of P288.00/box (24 capsules/box). It is currently available in Southstar Drug and soon to be available in Mercury Drug and Watsons stores.
